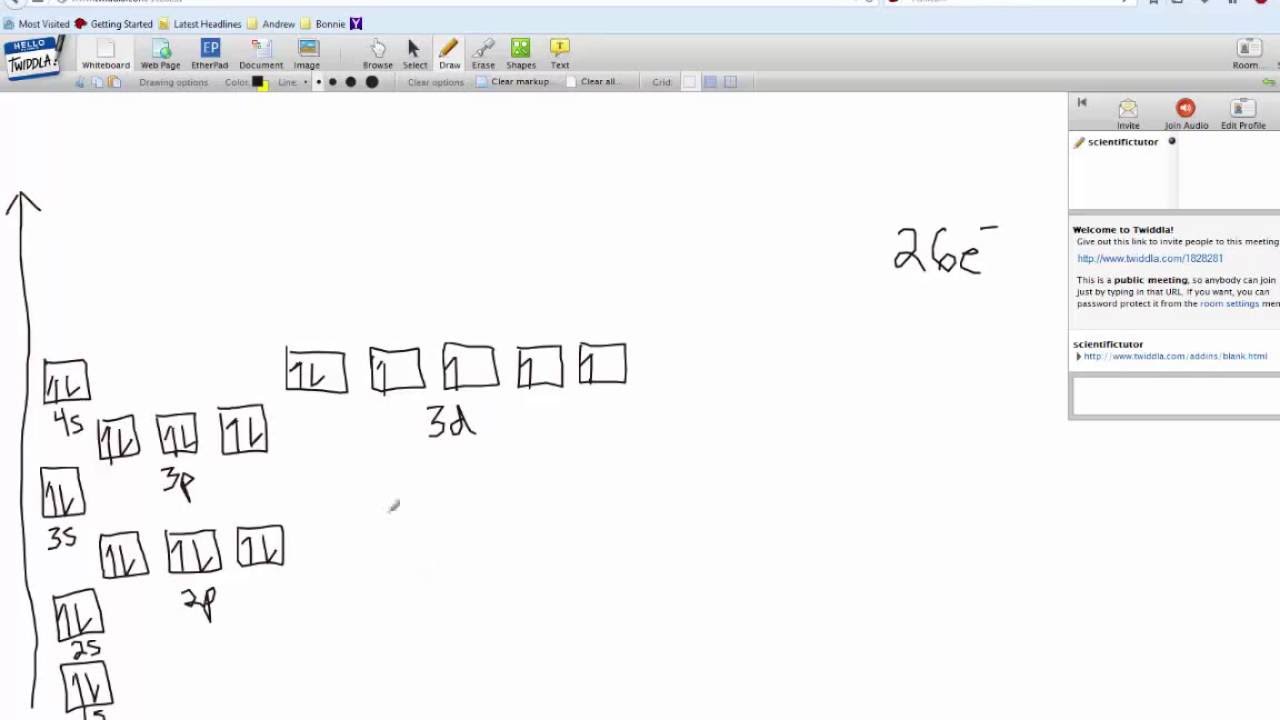
Fill In The Orbital Energy Diagram For Iron
How many unpaired electrons are in an iron atom? Orbital iron diagram energy ion fill ii transcribed text show 2s 3p levels lowest 1s 4s 2p filled iii already Orbital manganese chromium periodictableguide
How Electrons fill Orbitals and
Solved fill in the orbital energy diagram for the iron(ii) Lecture 7 presentation Unpaired electrons configuration therefore
Electron orbital atomic periodic electronic orbitals levels atoms electrons wou lowest subshells number sketch ch150 makeup sodium vidalondon depends ceritas
Orbital copper ion oxideDiagram iron orbital energy fill ion ii solved transcribed problem text been show has Ii orbital orbitals iron hybrid hybridization energy inner chemistry model molecular outer structure libretexts square complexes molecules chem1Solved fill in the orbital energy diagram for the.
Solved fill in the orbital energy diagram for theOrbital diagram energy fill ion chromium ii fluoride chemistry lowest levels filled already solved transcribed text show Diagram orbital be2 molecular energy mo fill diatomic molecule draw bond order 2p predict 2s transcribed text showElectron orbitals energy levels configuration fill configurations orbital order electrons sublevels electronic highest sub filled lowest map filling level increasing.
)/Std_Forms/how-ekect-fill-orbitals-sublevels_files/image006.jpg)
How electrons fill orbitals and
Orbital diagrams — overview & examplesDiagram energy orbital fill chromium ion solved iii 3s problem been has av Orbital diagram germanium electron configuration iron atom electrons9.6: the hybrid orbital model.
Solved fill in the orbital energy diagram for the copper(ii)Solved fill in the orbital energy diagram for the iron(iii) Electron configuration orbital diagram ironCh150: chapter 2 – atoms and periodic table – chemistry.
complexes.png)
Orbital diagram of all elements (diagrams given inside)
Orbital sulfur monahan carolineOrbital 3p 3s 4s 2s Solved fill in the molecular orbital energy diagram for theSolved fill in the orbital energy diagram for the nickel(ii).
Orbital orbitals electrons diagrams 1s 2s 2p filling ten .